Treatments
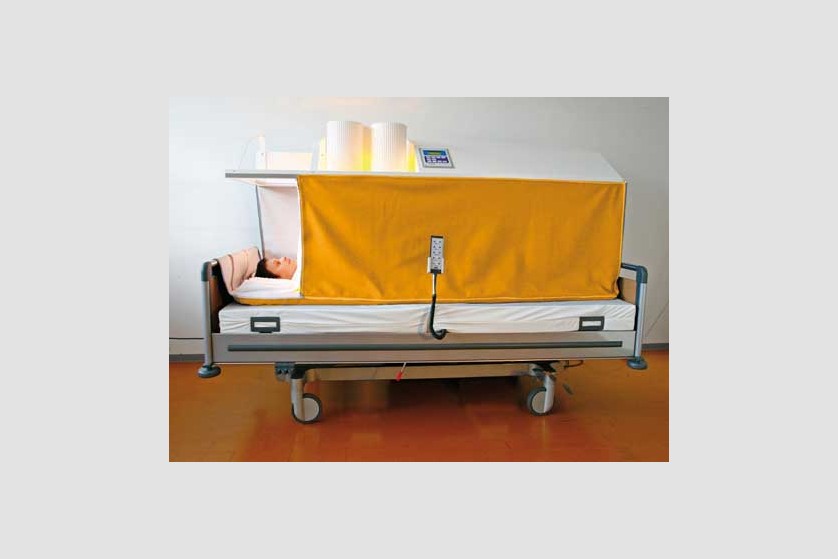
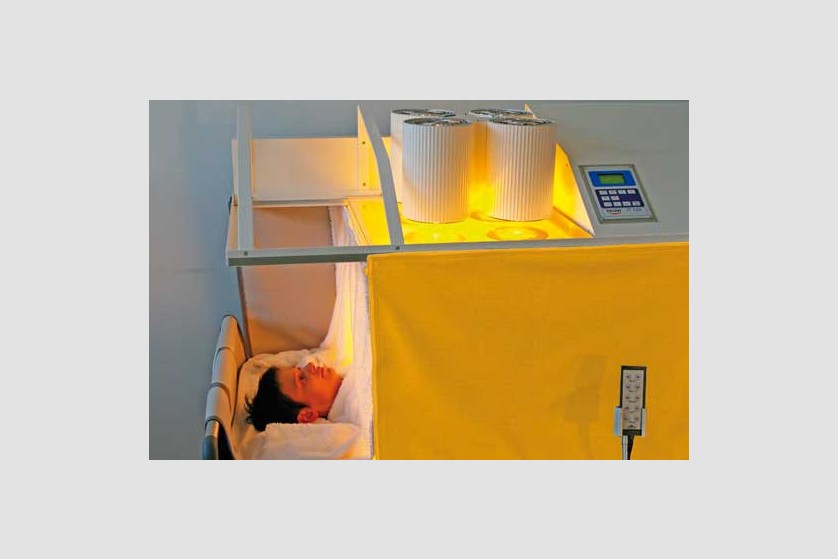
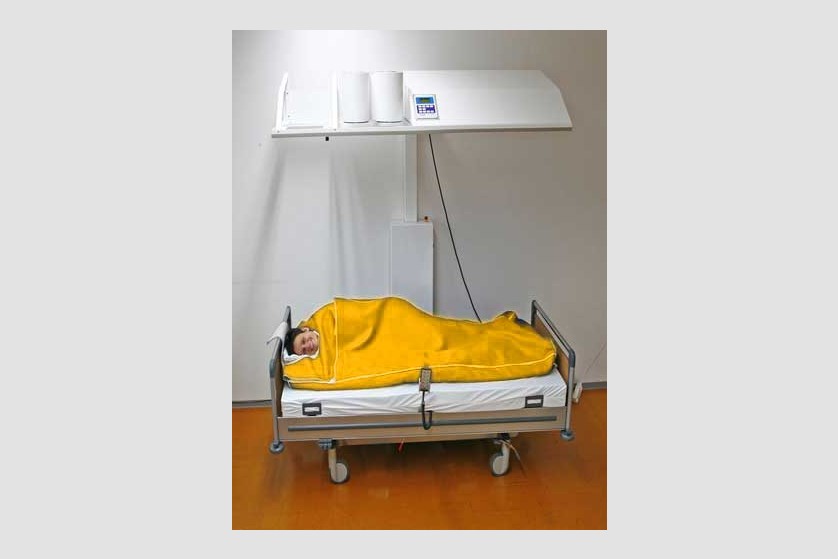
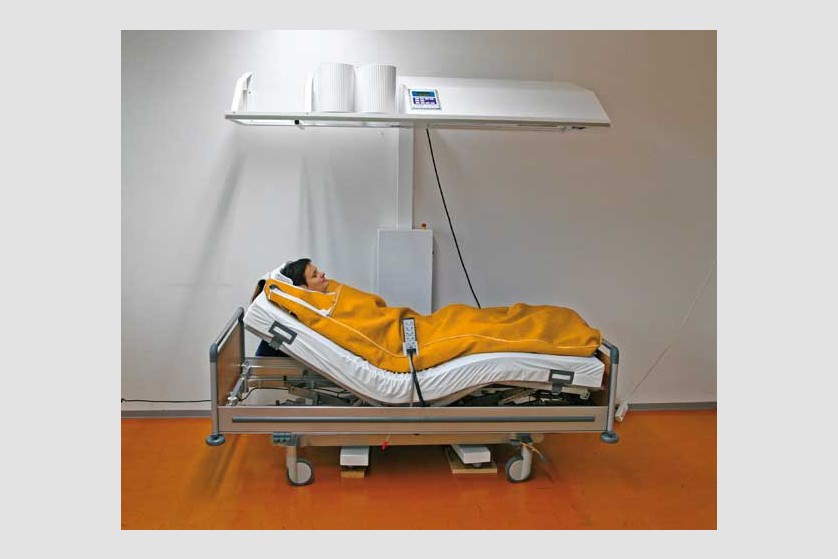
Hyperthermia
Hyperthermia is also called thermotherapy. This non-invasive form of therapy involves exposing the entire body or certain areas of the body to high temperatures for therapeutic purposes. In the past few years, this therapy has started being increasingly used as a complementary form in treating cancer.
Currently, general (systemic) and local hyperthermia are commonly used in several university medical centers in Europe and the United States.
Currently, general (systemic) and local hyperthermia are commonly used in several university medical centers in Europe and the United States.
How can you become a patient of our clinic?
Throughout the whole process, from your initial contact, through treatment and after you leave our clinic, our patient coordinators will guide you through the steps and support you with all their expertise, attention and kindness.
*
We are here to help you
Our patient coordinator will contact you soon
Phone: +40.771.518.946, e-mail: office@imuno-medica.ro