Electrochemotherapy
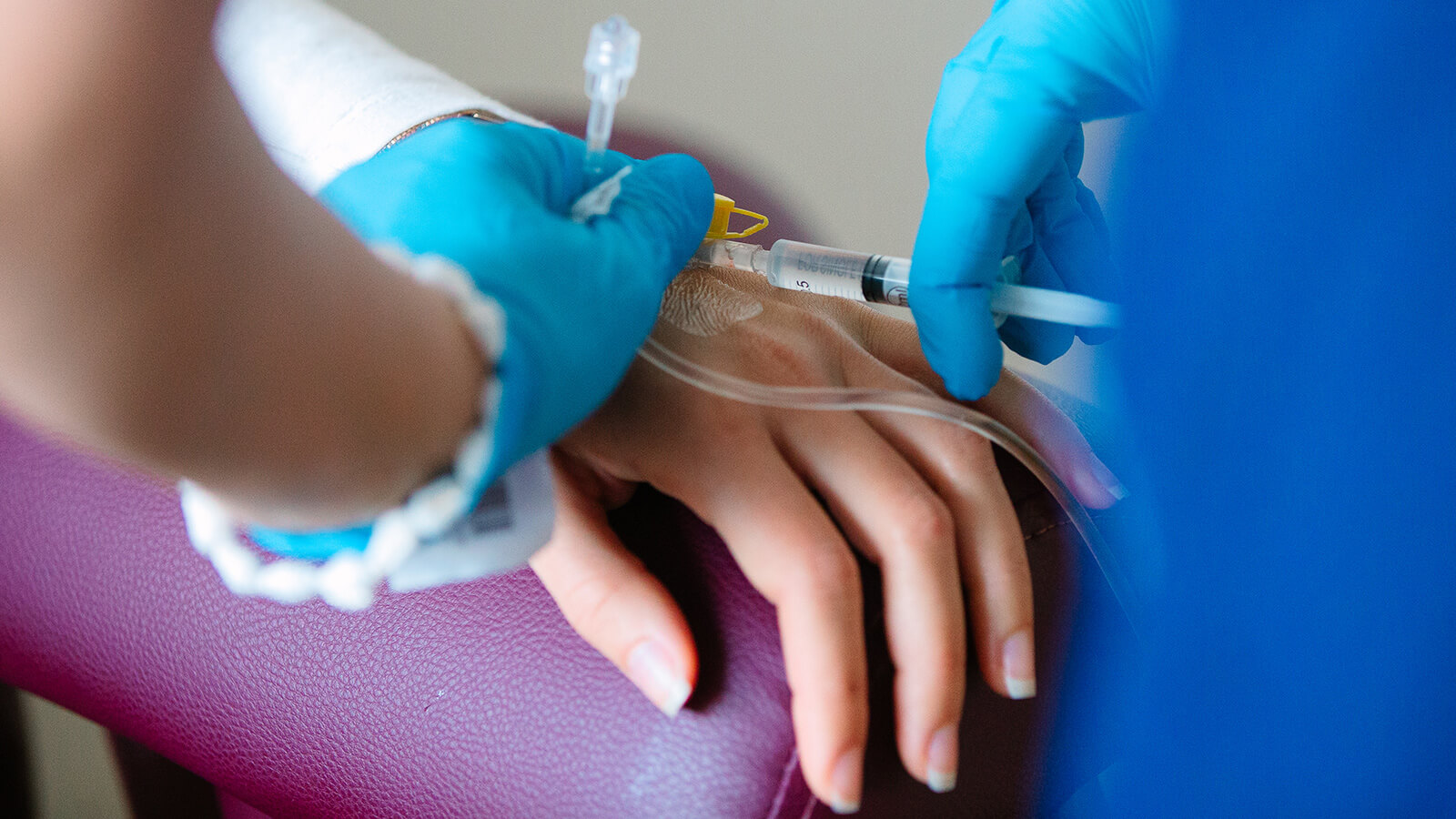
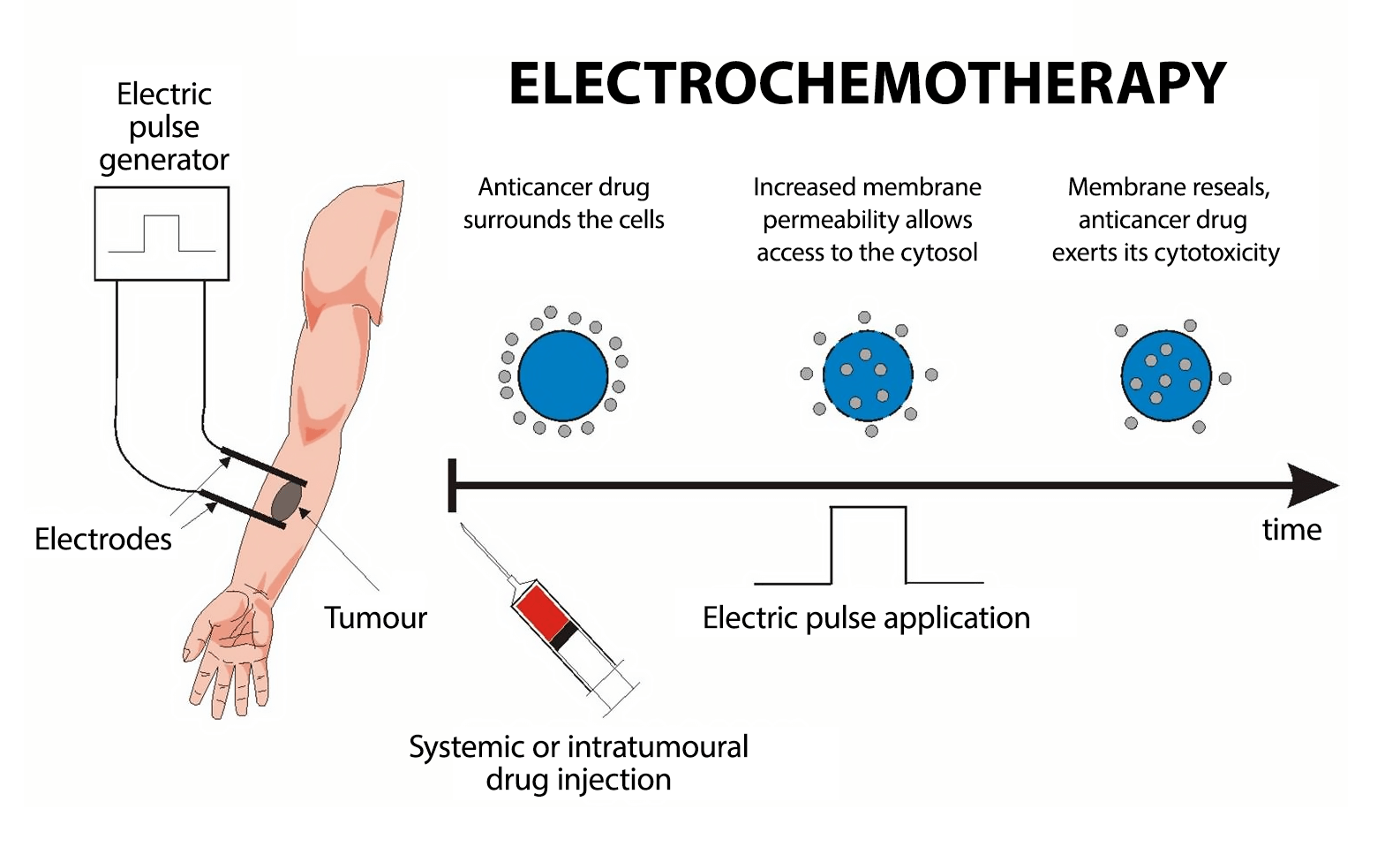
Electrochemotherapy (ECT), a state-of-the-art cancer therapy, employs electroporation — a method where electrical pulses significantly enhance the efficacy of the cytostatic agent by up to 10,000 times. This process facilitates the agent's substantial penetration into tumor cells.
Typically requiring just one or two sessions, this technique is exceptionally safe and, crucially, lacks the negative side effects associated with conventional chemotherapy.
Through electroporation, the electric current heightens the permeability of cell membranes, enabling the drug to infiltrate the cell and exert its toxic effects, resulting in cellular apoptosis and ultimately leading to tumor destruction.
Why electrochemotherapy
Electrochemotherapy stands out as one of the most effective, cost-efficient, and minimally invasive treatments in oncology. This technique involves administering cytostatic drugs either directly into the tumor or into the bloodstream, followed by the application of electric pulses. This process, termed electroporation, specifically targets cancer cells. A specialized probe delivers an electrical impulse to the malignant tumor, modifying the cancer cell membrane to create pores. These pores facilitate the entry of the medication, enhancing its effectiveness exclusively on cancer cells.
In preclinical studies, bleomycin and cisplatin have emerged as the drugs of choice for clinical applications in electrochemotherapy. The introduction of electric pulses significantly escalates the cytotoxic power of bleomycin, by nearly 10,000 times. The delivery of these pulses to tumors can be achieved using either plate electrodes placed on the skin above the tumor or needle electrodes inserted directly into it. The application of these electric pulses, irrespective of the electrode type used, has little to no impact on healthy tissue, ensuring a targeted approach to tumor treatment.
The Efficacy of
Electrochemotherapy
in Cancer Treatment
Electrochemotherapy has been demonstrated to be more effective compared to just administering cytostatic drugs. This method holds promise for treating malignant tumors of both skin and non-skin origins. Research indicates that small tumors have a response rate that is twice as high as that of larger tumors. Additionally, electrochemotherapy is a cost-effective, quicker, and more bearable approach for treating various tumor types. This is because the use of lower doses of cytostatic drugs leads to fewer side effects and less discomfort.
In terms of objective response rate (ORR), electrochemotherapy achieves over 80%, with a substantial proportion (53-86%) of these responses being complete and enduring. Recent data suggests an average effectiveness of around 84% for electrochemotherapy. In contrast, systemic chemotherapy, especially for stage four cancers, often serves a palliative function with response rates falling below 25% when used as monotherapy. Thus, the enhanced delivery of chemotherapeutic agents into tumor cells via electroporation emerges as a critical and potentially life-saving strategy. This method achieves significantly higher intracellular concentrations of bleomycin or cisplatin in tumor cells, using far smaller doses than traditional chemotherapy. This not only boosts their antitumor effectiveness but also preserves the integrity of normal cells, which are no longer adversely affected as they might be in conventional chemotherapy.
Protocol
Drug Administration
The treatment begins with the administration of a chemotherapeutic agent, typically bleomycin or cisplatin, to the patient. These agents are notably more effective when used in conjunction with electric field exposure.
Electroporation
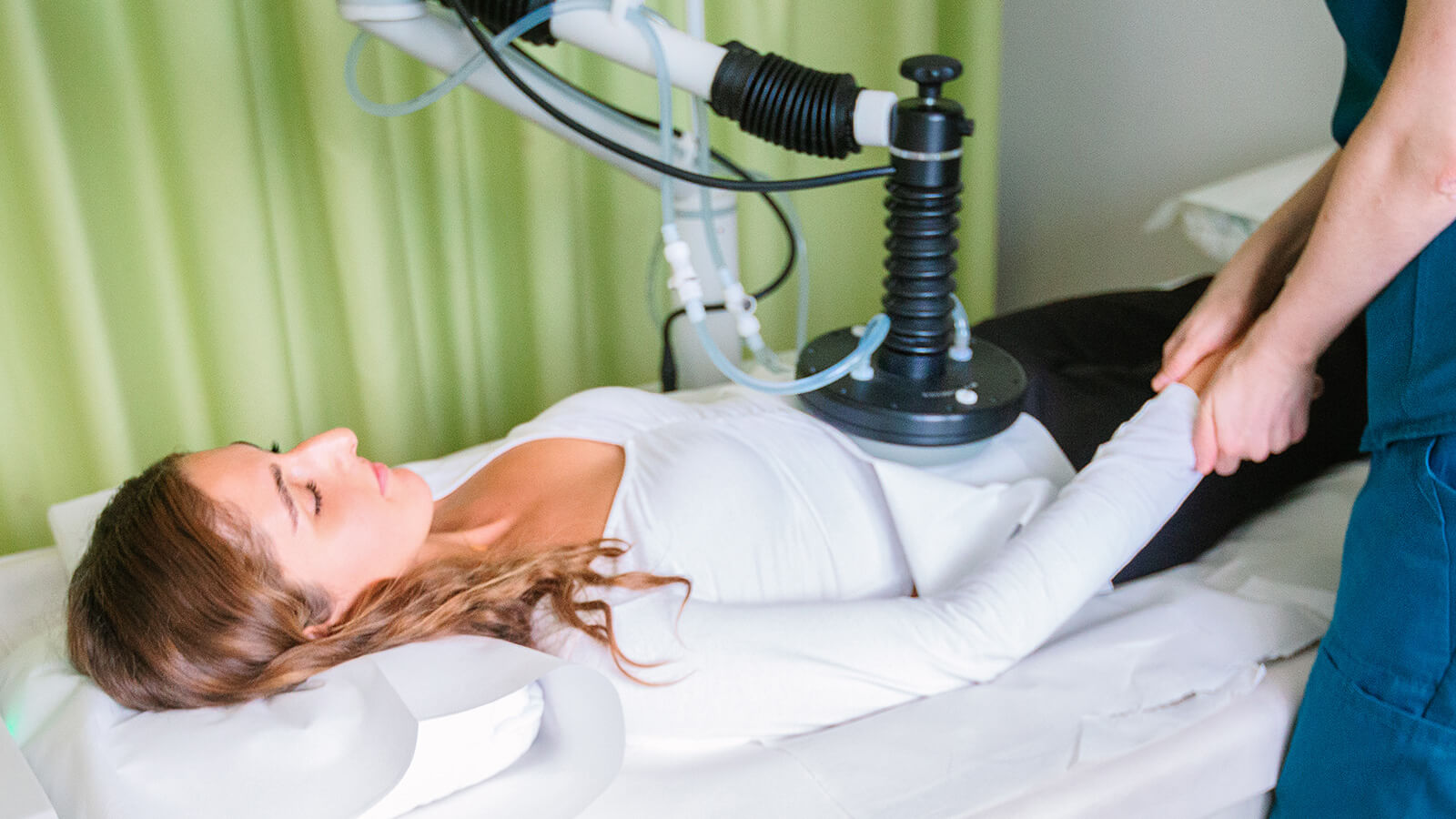
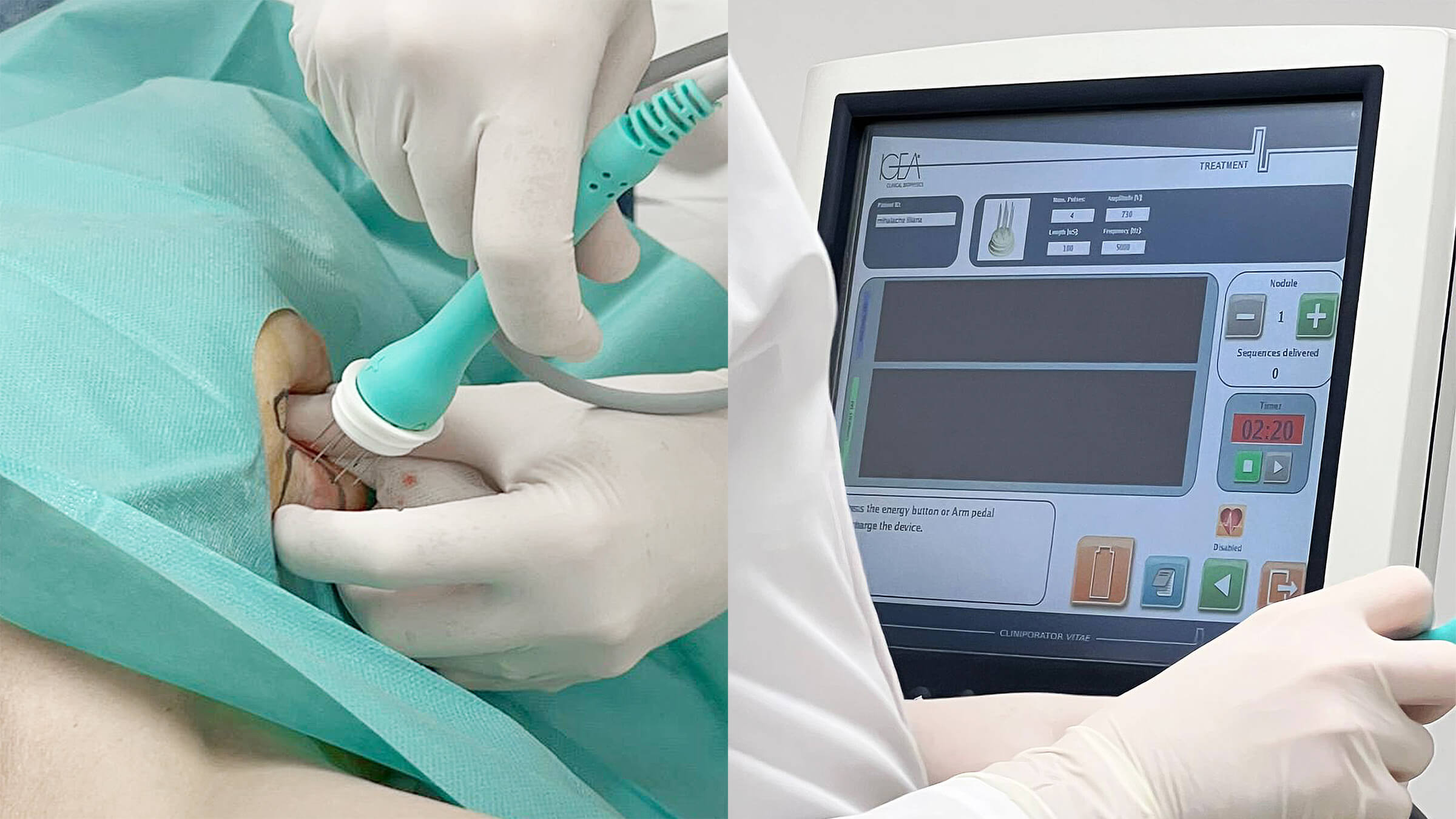
Eight minutes following the drug administration, specialized electrodes are strategically positioned either around or directly within the tumor tissue. The delivery of the electric pulse is facilitated by the CLINIPORATOR device, using a pen-like probe fitted with an electrode. The physician carefully inserts this electrode into the tumor, initiating the electric pulse. Each pulse lasts for 100 microseconds (µs), with a variable count ranging from 1 to 20 pulses. The amplitude of these pulses can vary between 100 and 1000 volts, with a frequency spectrum spanning from 1 to 5000 Hz. Throughout the procedure, the voltage and current profiles are continuously displayed on a monitor, providing real-time feedback and enabling the precise monitoring of each electroporation's effectiveness 1.
These electric pulses momentarily generate pores in the cell membranes, a process known as electroporation. This action significantly enhances the drug's penetration into the tumor cells, inducing tumor cell apoptosis. The duration of the treatment varies, lasting anywhere from 30 to 60 minutes, depending on the number of tumors being treated.
Advantages of Using
Electrochemotherapy
This technique notably enhances the concentration of chemotherapeutic drugs within the tumor tissue, allowing for the use of much smaller drug doses. This reduction in dosage significantly minimizes side effects. The primary benefit of electrochemotherapy lies in its ability to spare healthy tissue, setting it apart from traditional oncological treatments.
Electrochemotherapy is compatible with various other treatments like chemotherapy, radiotherapy, or supportive therapies, without causing any interference. In fact, it enhances the efficacy of all other cancer treatments. When the immune system is robust, undergoing electrochemotherapy can yield considerable advantages. In certain instances, this can result in the total elimination of the tumor and effectively halt the spread of metastases.
It is effective in reducing tumor masses, shrinking affected lymph nodes, alleviating local pain, and thereby improving the patient's quality of life. Furthermore, it offers a viable option for treating tumors that are resistant to other therapies and can be integrated into combined treatment plans.
European Approval of Electrochemotherapy
In use for over 25 years across Europe, electrochemotherapy is supported by numerous clinical studies and has even been incorporated into European treatment guidelines. It is primarily used with bleomycin and cisplatin for treating cutaneous metastases from various tumors and inoperable primary tumors.
Effects of
Electroporation
- Targeted destruction of tumor tissues
- Significant and irreversible damage to blood vessels supplying the tumor, leading to tumor cell death 2
- Induction of an immunogenic response capable of eradicating distant metastases through the abscopal effect
How Many Sessions
Are Needed?
Typically, a single session is effective. This session can stimulate an abscopal immunogenic response, where the immune system attacks other metastases. However, for larger tumors, a second session might be necessary.
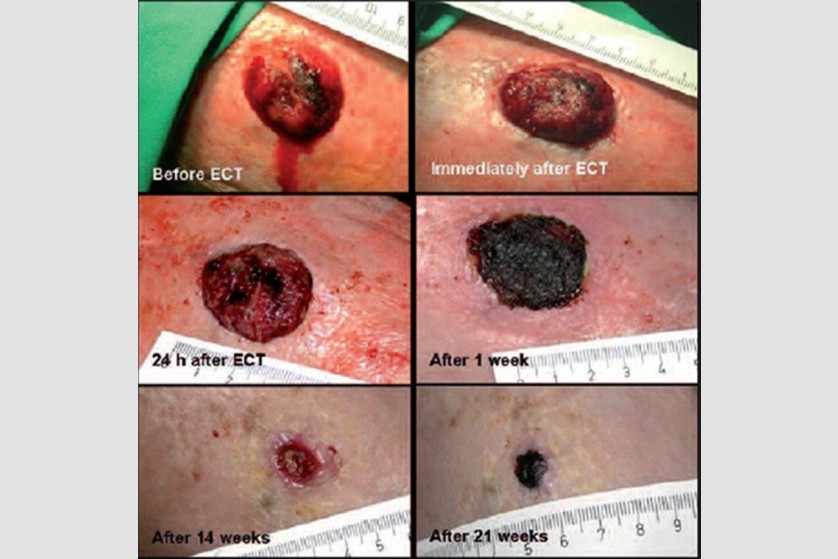
Through the courtesy of Dr. Gregor Sersa
What are the cancers
where electrochemo
therapy is indicated?
- Melanoma 3, especially recurrent or metastatic
- Skin cancer, such as basal cell carcinoma 4 and squamous cell carcinoma 5
- Breast cancer 6, particularly in cases that have proven resistant to other treatments recommended by guidelines. Studies show a complete response in 50% of cases, partial response in 21%, stable disease in 18%, and progression in only 8% of cases 7
- Recurrence at the chest wall in breast cancer, where a complete cure rate of 59% is recorded and an objective response rate of 89% is even higher than on primary tumors in breast cancer
- Bone metastases 8, where studies have recorded a response rate of 29%, stable disease in 59%, and progression in only 16%. The mineral structure of the bone and its regenerative capacity are not affected
- Sarcoma 9 can be treated by electrochemotherapy, especially when located in the skin or soft tissues
- Squamous cell carcinoma of the oral cavity and oropharynx 10
- Head and neck tumors 11, or those located at this level
- Rectal cancer 12
- Cutaneous metastases 13, of any histology, which are symptomatic due to bleeding, ulceration, breathing, smell, or pain
- Primary mucosal cancers 14, including recurrent tumors, where other treatment modalities (surgery, radiotherapy, systemic therapies) have failed or are not possible
- Primary unresectable pancreatic cancer 15
- Liver metastases 16 from solid tumors
- Cholangiocellular carcinoma 17 at the hepatic hilum
- Hepatocellular carcinoma 18
- Salivary gland cancer 19
- Esophageal cancer 20
Contraindications for electrochemotherapy
- Allergy or hypersensitivity to bleomycin or Cisplatin;
- Cumulative dose of bleomycin of 400,000 IU;
- Peripheral neuropathy > grade 2;
- History of pulmonary fibrosis will prevent intravenous administration of bleomycin;
- Infection and/or heart failure and/or liver failure and/or other serious systemic diseases;
- Severe uncorrectable coagulation disorders;
- Pregnancy, breastfeeding: potentially fertile patients must use appropriate contraceptive methods.
Benefits
Disease Control
- Objective response rate greater than 80%
- High percentage (53-86%) of long-lasting complete responses.
Symptoms to be
mitigated by ECT
- Bleeding
- Discharge
- Pain
- Smell
Other advantage
- Safe, easy, and extremely effective treatment
- Improvement in the patient's quality of life, regardless of life expectancy
- Positive cosmetic and functional results
- Electrochemotherapy is independent of histology
- Small doses of medication
- No significant side effects
- Can be safely repeated multiple times, and patients are willing to undergo repeat treatments
- Cost-effectiveness
Immunogenic Effect
of
Electrochemotherapy
Electrochemotherapy not only demonstrates a direct cytotoxic effect on cancer cells but also incites a systemic anticancer immune response through the activation of immunogenic cell death. This positions electrochemotherapy as a local immune response initiator, functioning akin to an in-situ vaccination with body-wide immunogenic impacts. The result is a more comprehensive and enduring antitumor effect, extending even to metastases that haven't undergone electroporation.
Consequently, a robust immune response is pivotal in this therapy, amplifying the immunogenic effects of electrochemotherapy 21. This encompasses both a sufficient quantity of immune cells, including T cells, NK cells, and Dendritic cells, and the integration of specific immune-boosting therapies. Research indicates a significant synergistic effect of electrochemotherapy when combined with immunotherapy 22, particularly in immunogenic tumors like melanoma 23, breast cancer 24, and liver cancer 25.
The advantages of Electrochemotherapy
at the ImunoMedica clinic
Given the primary objective of our clinic – to enhance the immune response against tumors – at ImunoMedica, electrochemotherapy is found to be effective in most cases for the destruction of tumors and the eradication of metastases.
This therapy is administered only after detailed analyses that elucidate the patient's anti-tumor response.
- Induce an anti-tumor immune response through the polarization of Th1 and M1 type immunity
- Suppress immune inhibition effected by Treg cells and MDSC
- Foster an intratumoral milieu that facilitates the infiltration of immune cells
- Augment the count of immune cells, including T helper cells, cytotoxic T cells, NK cells, NKT cells, Dendritic cells, and M1 macrophages
- Stimulate the activity of anti-tumor immune cells
The ImunoMedica Clinic is the sole facility in Romania offering electrochemotherapy for cancer treatment.
We utilize cutting-edge equipment, which stands as the most effective in the realm of electroporation. The majority of clinical and preclinical studies in electrochemotherapy have been conducted with our IGEA equipment.
To determine your suitability for electrochemotherapy in treating your specific cancer, we invite you to schedule a consultation at our clinic with Dr. Valentin Popescu, a leading expert in this field in Romania.
Bibliography
2. Jarm, Tomaz, et al. "Antivascular effects of electrochemotherapy: implications in treatment of bleeding metastases." Expert review of anticancer therapy 10.5 (2010): 729-746.
4. Clover, A. J. P., et al. "Electrochemotherapy for the treatment of primary basal cell carcinoma; A randomised control trial comparing electrochemotherapy and surgery with five year follow up." European Journal of Surgical Oncology 46.5 (2020): 847-854.
2. Jarm, Tomaz, et al. "Antivascular effects of electrochemotherapy: implications in treatment of bleeding metastases." Expert review of anticancer therapy 10.5 (2010): 729-746.
3. Sersa, Gregor. "The state-of-the-art of electrochemotherapy before the ESOPE study; advantages and clinical uses." European Journal of Cancer Supplements 4.11 (2006): 52-59.
4. Clover, A. J. P., et al. "Electrochemotherapy for the treatment of primary basal cell carcinoma; A randomised control trial comparing electrochemotherapy and surgery with five year follow up." European Journal of Surgical Oncology 46.5 (2020): 847-854.
5. Tokmakçı, Mahmut. "A high-voltage pulse generation instrument for electrochemotherapy method." Journal of medical systems 30 (2006): 145-151.
6. Wichtowski, Mateusz, et al. "Electrochemotherapy in breast cancer-discussion of the method and literature review." Breast Care 12.6 (2017): 409-414.
7. Matthiessen, Louise Wichmann, et al. "Electrochemotherapy for breast cancer—results from the INSPECT database." Clinical Breast Cancer 18.5 (2018): e909-e917;
Sersa, Gregor, et al. "Electrochemotherapy of chest wall breast cancer recurrence." Cancer Treatment Reviews 38.5 (2012): 379-386.
8. Bianchi, Giuseppe, et al. "Electrochemotherapy in the treatment of bone metastases: a phase II trial." World Journal of surgery 40 (2016): 3088-3094; Campanacci, Laura, et al. "Electrochemotherapy is effective in the treatment of bone metastases." Current Oncology 29.3 (2022): 1672-1682; Bocchi, Maria Beatrice, et al. "Electrochemotherapy in the Treatment of Bone Metastases: A Systematic Review." Journal of Clinical Medicine 12.19 (2023): 6150.
9. Campana, Luca G., et al. "Electrochemotherapy treatment of locally advanced and metastatic soft tissue sarcomas: results of a non-comparative phase II study." World journal of surgery 38 (2014): 813-822.
10. Prospective randomised trial with control group ‘Electrochemotherapy as first-line treatment of squamous cell carcinoma recurrences of the oral cavity and oropharynx: a randomised controlled trial’.EudraCT 2018-003589-15. Lead researcher: Dr Franco Ionna, G. Pascale Foundation National Institute of Tumours IRCCS, Naples.
11. De Virgilio, Armando, et al. "Electrochemotherapy in head and neck cancer: A review of an emerging cancer treatment." Oncology letters 16.3 (2018): 3415-3423; Strojan, Primož, et al. "Electrochemotherapy in mucosal cancer of the head and neck: a systematic review." Cancers 13.6 (2021): 1254.
12. Prospective randomised trial with control group ‘Organ sparing for locally advanced rectal cancer after neoadjuvant therapy followed by electrochemotherapy’. EudraCT number: 2018-004166-33. Lead Researcher: Dr Paolo Delrio, G. Pascale Foundation National Institute of Tumours IRCCS, Naples.
13. Matthiessen, Louise Wichmann, et al. "Management of cutaneous metastases using electrochemotherapy." Acta Oncologica 50.5 (2011): 621-629; Ferioli, Martina, et al. "Electrochemotherapy of skin metastases from breast cancer: a systematic review." Clinical & Experimental Metastasis 38 (2021): 1-10.
15. Prospective randomised trial with control group ‘Phase IIb trial to evaluate the efficacy of laparoscopic electrochemotherapy in the treatment of locally advanced pancreatic cancer’. EudraCT: 2018-003925-27; Phase I/II trial - feasibility and safety of a new surgical technology: electrochemotherapy in locally advanced pancreatic adenocarcinoma. Lead Researcher: Dr Francesco Izzo, G. Pascale Foundation National Institute of Tumours IRCCS, Naples.
16. Edhemovic, Ibrahim, et al. "Intraoperative electrochemotherapy of colorectal liver metastases." Journal of surgical oncology 110.3 (2014): 320-327; Edhemovic, Ibrahim, et al. "Intraoperative electrochemotherapy of colorectal liver metastases: a prospective phase II study." European Journal of Surgical Oncology 46.9 (2020): 1628-1633.
17. Tarantino, Luciano, et al. "Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: a feasibility study." European Journal of Surgical Oncology 44.10 (2018): 1603-1609; Granata, V., et al. "Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: a case report." European Review for Medical & Pharmacological Sciences 24.12 (2020).
18. Djokic, Mihajlo, et al. "Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study." European Journal of Surgical Oncology 44.5 (2018): 651-657.
19. Strojan, Primož, et al. "Electrochemotherapy in mucosal cancer of the head and neck: a systematic review." Cancers 13.6 (2021): 1254.
20. Egeland, Charlotte, et al. "Endoscopic electrochemotherapy for esophageal cancer: a phase I clinical study." Endoscopy International Open 6.06 (2018): E727-E734.
21. Calvet, Christophe Y., et al. "Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells." Oncoimmunology 3.4 (2014): e28131.
22. Calvet, Christophe Y., and Lluis M. Mir. "The promising alliance of anti-cancer electrochemotherapy with immunotherapy." Cancer and Metastasis Reviews 35 (2016): 165-177.
23. Goggins, Clare A., and Amor Khachemoune. "The use of electrochemotherapy in combination with immunotherapy in the treatment of metastatic melanoma: A focused review." International Journal of Dermatology 58.8 (2019): 865-870.
24. Denkert, Carsten. "The immunogenicity of breast cancer—molecular subtypes matter." Annals of Oncology 25.8 (2014): 1453-1455; Loizides, Sotiris, and Anastasia Constantinidou. "Triple negative breast cancer: Immunogenicity, tumor microenvironment, and immunotherapy." Frontiers in Genetics 13 (2023): 1095839.
25. Trotovšek, Blaž, et al. "New era of electrochemotherapy in treatment of liver tumors in conjunction with immunotherapies." World Journal of Gastroenterology 27.48 (2021): 8216; Trotovsek, Blaz, et al. "Laparoscopic electrochemotherapy for the treatment of hepatocellular carcinoma: Technological advancement." Frontiers in Oncology 12 (2022): 996269.
The therapeutic solutions we provide
Comprise a wide range of conventional, adjuvant and supportive therapies, which integrate medical concepts that have been built on a sturdy scientific basis and on the clinical experience of numerous cancer specialists worldwide.
ImunoMedica patients have access to the latest diagnostic tools, technologies and innovations as well as to the latest and best treatments available, as soon as these are proven to be safe and effective.
How can you become a patient of our clinic?
Throughout the whole process, from your initial contact, through treatment and after you leave our clinic, our patient coordinators will guide you through the steps and support you with all their expertise, attention and kindness.
*
We are here to help you
Our patient coordinator will contact you soon
Phone: +40.771.518.946, e-mail: office@imuno-medica.ro