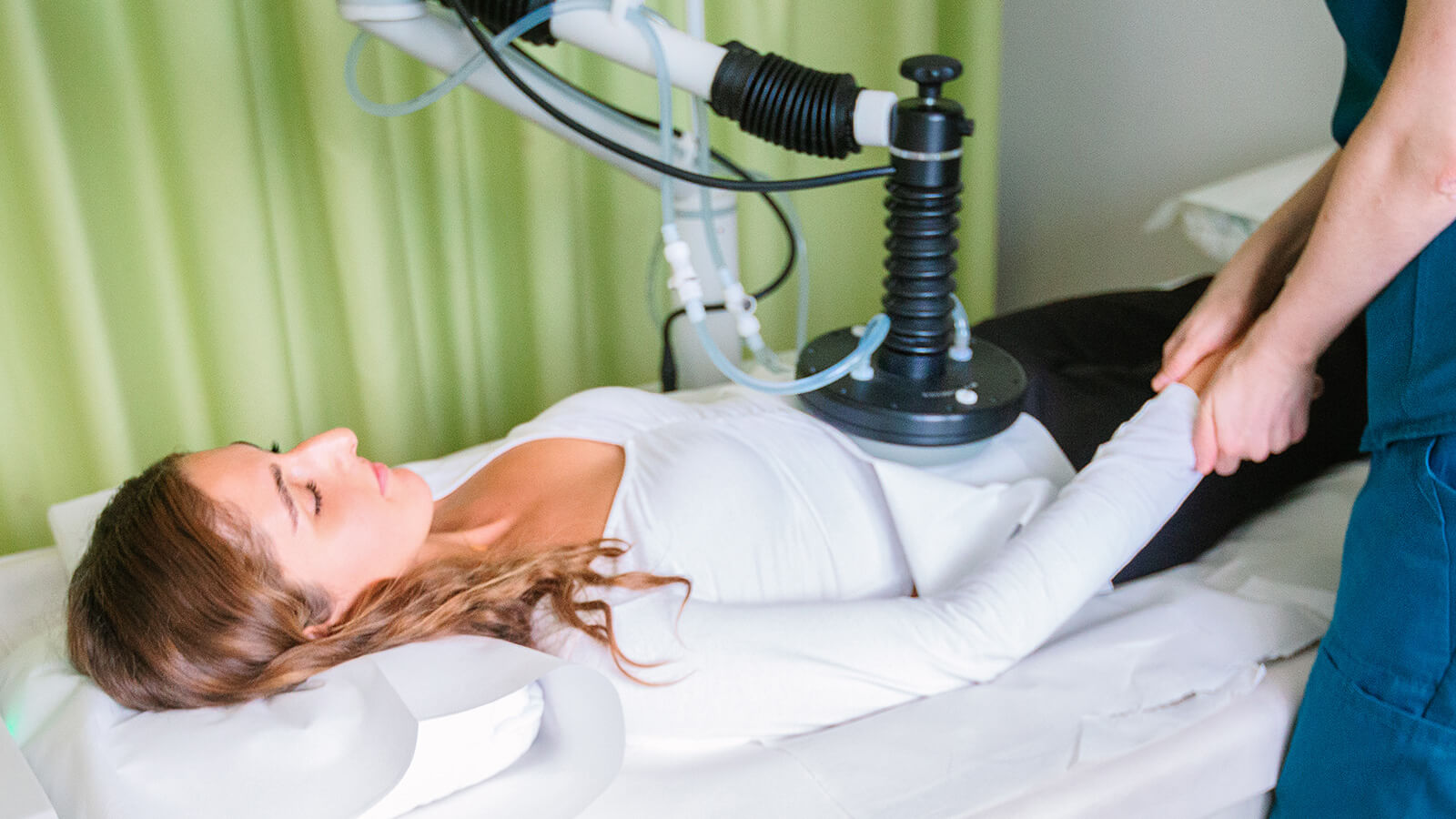
Hyperbaric oxygen in cancer
It is a well known fact that hypoxia is a common feature of different solid tumors. Local impairment of the circulatory system entails the generation of a heterogeneous population of tumor cells that are not normally supplied with oxygen and nutrients in most regions of the tumor, except those that are in close proximity to the blood vessels. The reduced cell division seen in areas with low oxygenation in tumors results in resistance to both radiotherapy and chemotherapy.
rly vascularized areas of the tumor may be perfused, through sub-lethal levels, by cytotoxic agents, thus generating an acquired resistance to chemotherapy.
A study published in Nature magazine has also shown that hypoxia may be responsible for a variety of growth modulation effects that could generate an advantage of cancer cell growth.
Yang and his research team found that the hypoxia-induced expression of factor-1 (HIF-1), suggested to be an endogenous marker of tumor hypoxia, significantly affected the overall survival and the disease-free survival of osteosarcoma patients.

Antitumor effect of
hyperbaric oxygen
therapy (HBOT)
These features of hyperbaric oxygen therapy increase the partial pressure of oxygen inside the tumor and make it more susceptible to radiotherapy.
DRAMATICALLY INCREASES
the amount of oxygen dissolved in the plasma
OXYGENATES
the hypoxic tissues
PROMOTES
neovascularization, eventually leading to increased blood flow.
HBOT has been extensively and successfully used in radiotherapy to increase radiation sensitivity and tumor oxygenation. In the same way, hyperbaric oxygen therapy enhances the perfusion of chemotherapeutic agents into hypoxic tumors and the susceptibility of tumor cells to such drugs.
Several studies have suggested that hyperbaric oxygen therapy can promote the growth or recurrence of malignancy by promoting angiogenesis, while other studies have shown inhibitory effects on tumor growth. However, two independent research groups have recently reviewed experimental and clinical data from the literature of the past 50 years and concluded that intermittent exposure to HBOT had no stimulatory effects on primary or metastatic cancer growth.
Currently, HBOT is used clinically in combination with radiotherapy and chemotherapy as a treatment approach of malignant tumors.
Bibliography
Bush RS, Jenkin RD, Allt WE, et al: Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer 1978. 3: 302-306.
Teicher BA, Lazo JS and Sartorelli AC: Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res 1981. 41: 73-81.
Bush RS, Jenkin RD, Allt WE, et al: Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer 1978. 3: 302-306.
Brown JM: Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol 1979. 52: 650-656.
Teicher BA, Lazo JS and Sartorelli AC: Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res 1981. 41: 73-81.
Graeber TG, Osmanian C, Jacks T, et al: Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996. 379: 88-91
Yang QC, Zeng BF, Dong Y, et al: Overexpression of hypoxia-inducible factor-1alpha in human osteosarcoma: correlation with clinicopathological parameters and survival outcome. Jpn J Clin Oncol 2007. 37: 127-134
Vukovic V, Haugland HK, Nicklee T, et al: Hypoxia-inducible factor-1alpha is an intrinsic marker for hypoxia in cervical cancer xenografts. Cancer Res 2001. 61: 7394-7398
Valaitis j, Van Elk J and Staley CJ: Effect of hyperbaric oxygen and nitrogen mustard (NSC-762) on Ehrlich ascites tumor. Cancer Chemother Rep 1968. 52: 405-412.
Shewell J and Thompson SC: The effect of hyperbaric oxygen treatment on pulmonary metastasis in the C3H mouse. Eur J Cancer 1980 16: 253-259.
DeCosse JJ and Rogers LS: Influence of high-pressure oxygen and chemotherapy on the AMel 4 hamster melanoma. Cancer Res 1966. 26: 287-292.
Dettmer CM, Kramer S, Gottlieb SF, Aponte GE and Driscoll DH: The effect of increased oxygen tensions upon animal tumor growth. Am J Roentgenol Radium Ther Nucl Med 1968. 102: 804-810.
Feldmeier J, Carl U, Hartmann K and Sminia P: Hyperbaric oxygen: does it promote growth or recurrence of malignancy? Undersea Hyperb Med 2003. 30: 1-18.
The therapeutic solutions we provide
Comprise a wide range of conventional, adjuvant and supportive therapies, which integrate medical concepts that have been built on a sturdy scientific basis and on the clinical experience of numerous cancer specialists worldwide.
ImunoMedica patients have access to the latest diagnostic tools, technologies and innovations as well as to the latest and best treatments available, as soon as these are proven to be safe and effective.
How can you become a patient of our clinic?
Throughout the whole process, from your initial contact, through treatment and after you leave our clinic, our patient coordinators will guide you through the steps and support you with all their expertise, attention and kindness.
*
We are here to help you
Our patient coordinator will contact you soon
Phone: +40.771.518.946, e-mail: office@imuno-medica.ro